Boiling Point Definition
In our everyday life we compare people and things to express ourselves vividly. The most important essential in a discussion or matter.

Difference Between Normal Boiling Point And Standard Boiling Point Compare The Difference Between Similar Terms

Boiling Point And Melting Point In Organic Chemistry Chemistry Steps

Ibp Definition Initial Boiling Point Abbreviation Finder
By definition one of the.

Boiling point definition. The freezing point and boiling point of a pure solvent can be changed when added to a solution. A peninsula cape or promontory. If we change the external pressure from 1 atm lower or higher the boiling point changes as well.
Definition of Boiling Point. At any temperature a liquid partly vaporizes into the space above it until the pressure exerted by the. By definition a liquid boils when the vapor pressure of the gas escaping from the liquid is equal to the pressure exerted on the liquid by its surroundings as shown in the figure below.
A mark or dot used in printing or writing for. Very valuable or costly. To explain why water boils at 90 o C in the mountains and 120 o C in a pressure cooker even though the normal boiling point of water is 100 o C we have to understand why a liquid boils.
It is currently defined by two fixed points. Temperature and Boiling point The vapor pressure of a substance is the pressure at which its gaseous vapor phase is in equilibrium with its liquid or solid phase. It is a measure of the tendency of molecules and atomsto escape from a.
A tapering extension of land projecting into water. Of a liquid heated to the point when it starts to turn into a gas. Learn about the uses and methods of boiling.
Simmering is gentle boiling while in poaching the cooking liquid moves but scarcely bubbles. Boiling the cooking of food by immersion in water that has been heated to near its boiling point 212 F 100 C at sea level. Boiling point definition Boiling point is simply the temperature at which water starts boiling in other words it changes its state from liquid to gas.
Boiling point elevation like freezing point depression is a colligative property of matter. The boiling point of water is typically considered to be 100 C or 212 F. Boiling point temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapour of the liquid.
Here T BP is the boiling point elevation-- the change in the boiling point that occurs when a solute dissolves in the solvent and k b is a proportionality constant known as the molal boiling point elevation constant for the solvent. This means it depends on the number of particles present in a. DeltaT_f -K_f times m DeltaT_f K_b times m.
When this occurs the freezing point of the pure solvent may become lower and the boiling point may become higher. The temperature at which a liquid boils and turns into a gas. Vapor pressure temperature and boiling point For more information see.
Because the boiling point of a liquid rises with pressure the contents of the pressurized vessel can remain liquid so long as the vessel is intact. Boiling is the method of cooking food in boiling water or other water-based liquids such as stock or milk. In 1982 the International Union of Pure and Applied Chemistry IUPAC0 defined the standard boiling point as the temperature of boiling under 1 bar of pressure.
Pressure and a change in the composition of the liquid may alter the boiling point of the liquid. An object having a sharp or tapered end. Water-soluble substances such as sugar and salt raise the boiling point.
Sometimes boiling point is defined by the pressure at which the measurement was taken. F is a unit of temperature that was widely used prior to metrication. Cc is a cubic centimeter and is equal to a mL Therefore.
Mathematically a per statement is translated as a division. The extent to which these changes occur can be found using the formulas. The formal definition of density is mass per unit volumeUsually the density is expressed in grams per mL or cc.
The boiling point temperature will be lower if the atmospheric pressure is decreased. Under this condition addition of heat results in the transformation of the liquid into its vapour without raising the temperature. Boiling definition having reached the boiling point.
Precious definition of high price or great value. The point of a knife. Mathematical Definition of Density.
The temperature at which water freezes 32F and the boiling point of water 212F both at sea level and standard atmospheric pressure. Comparison is a rhetorical or literary device in which a writer compares or contrasts two people places things or ideas. To reach or cause something to reach the temperature at which a liquid starts to turn into a.
A boiling liquid expanding vapor explosion BLEVE ˈ b l ɛ v iː BLEV-ee is an explosion caused by the rupture of a vessel containing a pressurized liquid that has reached temperatures above its boiling point. Types of Boiling Points Boiling point is also known as saturation temperature. The boiling point of different liquids is different for a given pressure.
By definition Boiling Point of a given substance is the temperature at which the vapor pressure of a liquid substance is equal to the surrounding. Noun the point at which a person gives way under stress. A mark formed by or as if by a sharp end.
The point of the antenna. For example the boiling point of pure water at standard atmospheric pressure or sea level is 100C 212F while at 10000 feet 3048m it is 9039 C 1947F. A sharp or tapered end.
Steaming or bubbling up under the action of heat. The temperature at which liquid vapour pressure equals atmospheric pressure is referred to as boiling point. A stone projectile point.
Boiling is used primarily to cook meats and vegetables. Noun an individual detail. The normal boiling point of a liquid is defined as the temperature at which the vapor pressure of the liquid is equal to standard pressure 1 atm.
This temperature is dependent on pressure and the substances latent Heat of vaporization. In 1982 IUPAC defined the standard boiling point of a liquid as the temperature at which the liquid boils under a pressure of 1 bar.
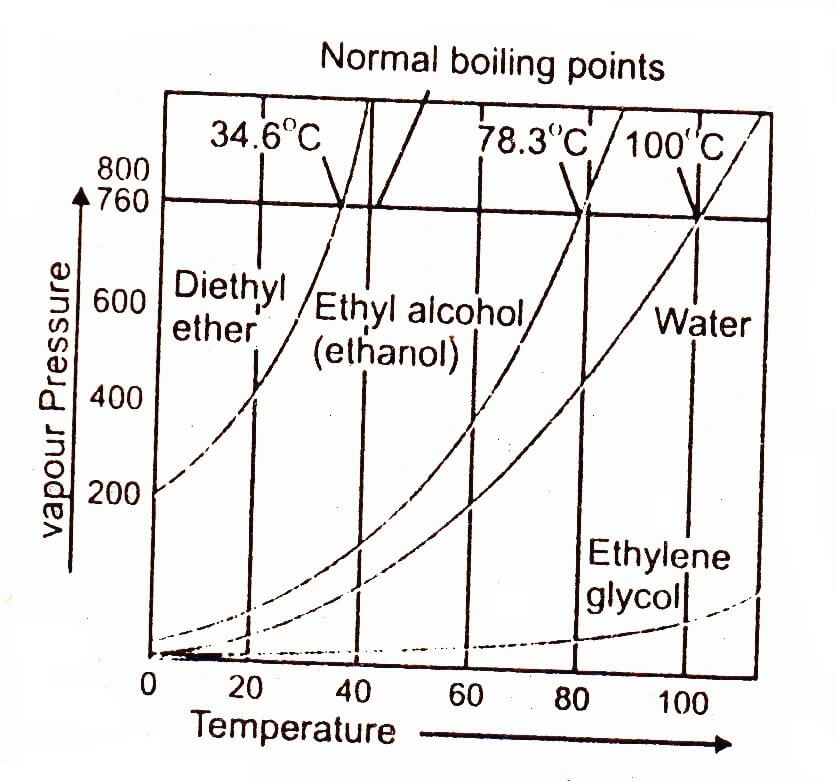
Definition And Explanation Of Boiling Point Chemistry Skills

Melting Point Freezing Point Boiling Point

Ppt Melting Point And Boiling Point Powerpoint Presentation Free Download Id 2326555

Boiling Point Elevation Chemistry For Non Majors

Determination Of Boiling Point Of Organic Compounds

How To Boil Water The Washington Post

Ppt Boiling Point Powerpoint Presentation Free Download Id 2402961

Differences Between Evaporation And Boiling In Chemistry
Comments
Post a Comment